Nickel is the fifth most commonly found metal on the earth, with a shiny appearance, excellent hardness, and corrosion-resistive properties, making it ideal for preventive coating on the surfaces of other materials. As a result, Nickel is the best plating option for various metals such as aluminum, steel, copper, tungsten, polymer, and many more. Electroless Nickel plating is a popular chemical plating process from a solution containing Nickel, sulfate, phosphate, and carbolic acid. These solutions are mixed and heated before proceeding with the plating. This process has been used in the manufacturing industry for over 50 years, so you can imagine how popular it is in surface finishing applications.
Parts with Electroless nickel plating
Solutions Used in Electroless Nickel Plating
There are various chemical solutions used for electroless nickel plating. Let’s understand each of them through the comparative study table below;
| SN |
Chemical
|
Role |
Examples
|
| 1 |
Soluble salt of Nickel
|
It gets reduced and deposited On the surface of the material to be coated(substrate)
|
Nickel chloride (NiCl₂), Nickel Sulfate (NiSO₄) |
| 2 |
Reducing Agent
|
It gets oxidized by reducing the metal ion.
|
Formaldehyde(CH 2 O ), Hypophosphite
|
| 3 |
Complexion agent
|
Improve the quality of nickel deposition
|
Fluorides, glycinates, succinates
|
| 4 |
Stabilizer
|
Prevent the decomposition of the plating bath
|
Thallium, Calcium
|
| 5 |
Buffer
|
Control the PH of the plating bath to obtain a thin and uniform deposition of Nickel
|
Sodium acetate, sodium hydroxide
|
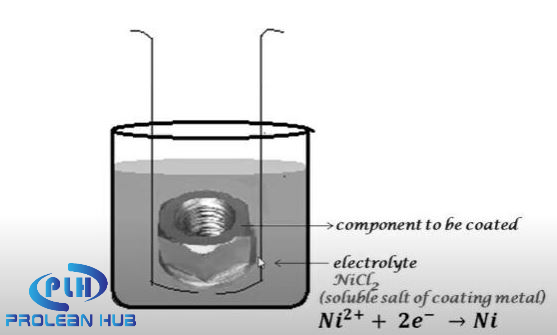
How does it Work?
Working principle
Here, electro-less means that no current is used in the plating process. Instead, the reducing agent supplies the electron for metal ion reduction, resulting in high throwing power. The nickel ion (2e +) in the solution, while reacting with the reducing agent (2e-), deposit the Nickel on the surface of the substrate material.
Ni2+ (from the nickel salt solution) +2e- (from the Reducing agent) = Ni (on the substrate surface)
Steps of Electroless Nickel Plating
Step 1: Preparing for the plating
The first step is to clean the parts to be coated so that any contamination, such as dust, oil, slags, greases, and any chemical on the surface, is washed away, preparing it for the best nickel coating adhesion. Where cleaning agents such as sulfuric acid and hydrochloric acid are used, it is washed with distilled water in a hot bath to neutralize the acid and prevent it from degrading the surface.
Step 2: Treatment on the plating bath
Treatment plant for electroless nickel plating
After cleaning the parts and preparing the bath solution, the plating process can begin. The parts are now immersed in a nickel-plating bath containing positive charges. The material to be coated attracts positively charged nickel ions to their surfaces, resulting in a fine layer of coating. Because electroless nickel plating does not require an electric source, the temperature of the plating bathing is the primary control variable in the process (70 to 900C is preferred).
The thickness of the deposited nickel layer varies between 5 and 25 microns per hour. However, because there is no current and it is an autocatalytic plating process, there is no such limit on thickness; as the treatment time increases, so will the deposition thickness.
Step3: Post-processing
During the post-processing stage, the attached particles and residues are removed by rinsing with an acid, alkali, and surfactant solution. Then, additional finishing, such as polishing, waxing, and others, are applied depending on the requirements.
Influencing Factors in Electroless Nickel Plating
Several factors influence the results of nickel electroless plating, which must be controlled to achieve the required surface finishing quality.
1. Imperfection on the surface
The nickel plating is affected by surface imperfections such as burrs, slags, and preformed rust. So the best idea is to keep these things in mind while machining and then remove the defects with the deburring process.
2. Cleanliness of the surface
Before proceeding with the nickel plating, the dust, oils, or soaps formed by the saponification of oil by an alkaline cleaner need to be removed. If the surface cleaning is not done correctly, the layer of Nickel may peel or become damaged after some time.
3. PH value
Maintaining the PH value is critical for uniform nickel plating using an electroless approach. The PH scale should be between 3.8 and 5. In the solution, it is expected that the pH tends to rise as time passes, so stabilizer and buffer solutions must be used to keep the PH stable throughout the treatment process.
As the PH rises, more hydroxide ions are formed, which bond with nickel ions and form nickel hydroxide, which has a light green color.
4. The concentration of Nickel ion
The salt solution used in the treatment surface is the nickel ion source deposited on the substrate surface. Therefore, if the nickel ion concentration is low, the plating process becomes slower. However, the too-high concentration is deposited faster, but the deposition will be un-uniform. The standard range of nickel concentration is between 20 and 45 g /l.
5. Temperature
The treatment bath temperature needs to be between 70 and 900C. As the temperature rises, some aromatic components of the additives will evaporate, requiring more time for the deposition.
Try Prolean Now!
Advantages
Like other plating and surface finishing approaches, the primary advantage is the coated parts and products become corrosion highly resistive and withstand the critical environmental effects. But, besides that, electroless nickel plating has many more benefits. Let’s look at some crucial advantages in detail.
Low cost
One of the cost factors in the plating finish approach is the cost of electricity. However, since electroless nickel plating does not need an electric source, it is more cost-effective than other methods, such as zinc plating.
Uniform coating
Electroless nickel plating results in a uniform coating on the substrate surface. Temperature, PH value of plating bath, the concentration of nickel ion, treatment time, and many other factors can all be controlled to achieve the desired thickness of nickel plating.
Dimensional consistency
Because up to 5-micron plating thickness is achievable, it does not affect the required tolerance on the parts.
Hardness
The plating bath’s phosphorous content influences the plated parts’ hardness. Low phosphorus levels increase hardness while decreasing the corrosion resistance of the plating layer.
Minor surface repairing
Electroless nickel plating also aids in the repair of minor cracks on the substrate surface. Therefore, it can be very beneficial to plate nooks, crannies, and blind gaps with uniform thickness.
Aesthetic and other benefits
The yellowish-white color of nickel plating offers appealing aesthetic beauty to the parts and products. In addition, it is less complex. It does not require a complicated filtration process. In today’s scenario, automated equipment is available in the manufacturing industry, making it easier to control the process.
Applications
The electroless nickel plating process contributes to various industries to make their components corrosion resistive and durable. Following are the key industries where nickel plating is used;
| SN | Industry | Applicable parts | What does it make? |
| 1 | Aerospace | valves, pistons, engine shafts covering the surface, engine mounts, compressor blades, and other flight-critical components | It has excellent wear resistance, corrosion resistance, chemical resistance, and lubricity, which is critical for aerospace parts that require a high degree of accuracy over a long period. |
| 2 | Automotive | Pistons, cylinders, gears, shafts, fuel injection system, rivets, thrust transmitters, knuckle pins, housing, and many more components | Provide wear protection and corrosion resistance |
| 3 | Hardware | Bathroom fixtures, Doorknob, handles Piping, and many more. | Corrosion resistance |
| 4 | Electrical & Electronics | Cover various equipment, Heat sinks, hard drive disks, Printed circuit boards | Protection from corrosion and environmental exposer |
| 5 | Oil & gas | Valves, pumps, pipe fittings, storage tanks, and others | Protection from corrosion and environmental & chemical exposure. |
Conclusion
Electroless nickel plating is a unique surface finishing method for many materials, including steel, copper, bronze, aluminum, plastic, and many others. Nickel plating improves durability by providing excellent corrosion resistance on the substrate surface. In addition, because Nickel has a shiny yellowish white color, it offers appealing aesthetic beauty. In addition, the plating can be done with or without electrolysis, check the difference between Electroplating and Electro-less plating.
Because there is a lot of chemical and catalytic reaction in the process, electroless nickel plating may be confusing for many people, especially those who do not have any professional knowledge of engineering chemistry. However, here at Prolean, our engineers and technicians have worked on surface finishing technology, including electroless nickel plating, for over a decade. Therefore, they will understand the proper chemical composition and approach once you explain your requirements and applications.
FAQ’s
What is electroless nickel plating?
It is one of the surface finishing methods in which a layer of Nickel is deposited on the substrate by means of catalytic chemical reactions & without using electricity.
What are the two prime solutions used in the electroless nickel plating?
A salt solution containing nickel ions & Reducing agent are the two primary chemical solutions used in the process.
What are the most common materials coated with electroless nickel plating?
This method is commonly used to coat alloy steel, stainless steel, aluminum copper, brass, bronze, and plastics.
What are the factors that affect the quality of nickel plating?
Temperature & PH of the plating bath, Treatment time, cleanness of substrate surface, and concentration of Nickel ion in the solution are primary factors influencing the plating outcome.







0 Comments